07 Oct_2022
FDA approval for LimaCorporate's novel novel TT glenoid replacement

As our Modular Shoulder System celebrates its 20th anniversary, we now have more reasons to be happy. We can anticipate that in 2023 we will have much to celebrate!
Today we received FDA Approval for our novel TT glenoid replacement that joins the recently approved shoulder platform to be launched in 2023!
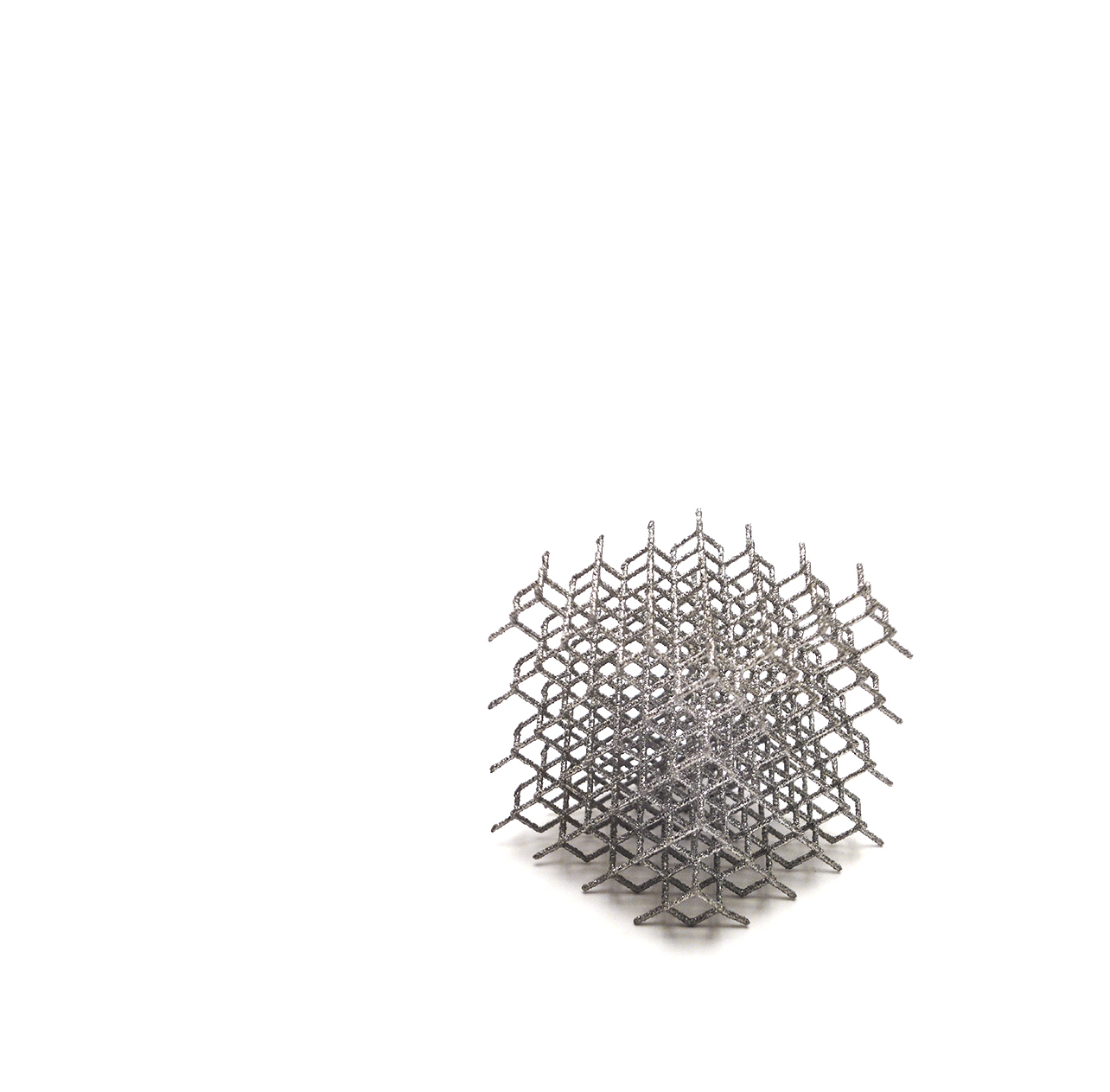
For the first time in the history of LimaCorporate, both the glenoid and humeral components are fully 3D Printed leveraging our long legacy in additive manufacturing.
This novel implant system takes advantage of the patented Trabecular Titanium (TT) technology.
We're still working behind the scenes, and we're sure that in 2023 this will be an exciting opportunity for LimaCorporate in the USA.
Read the full press release here.