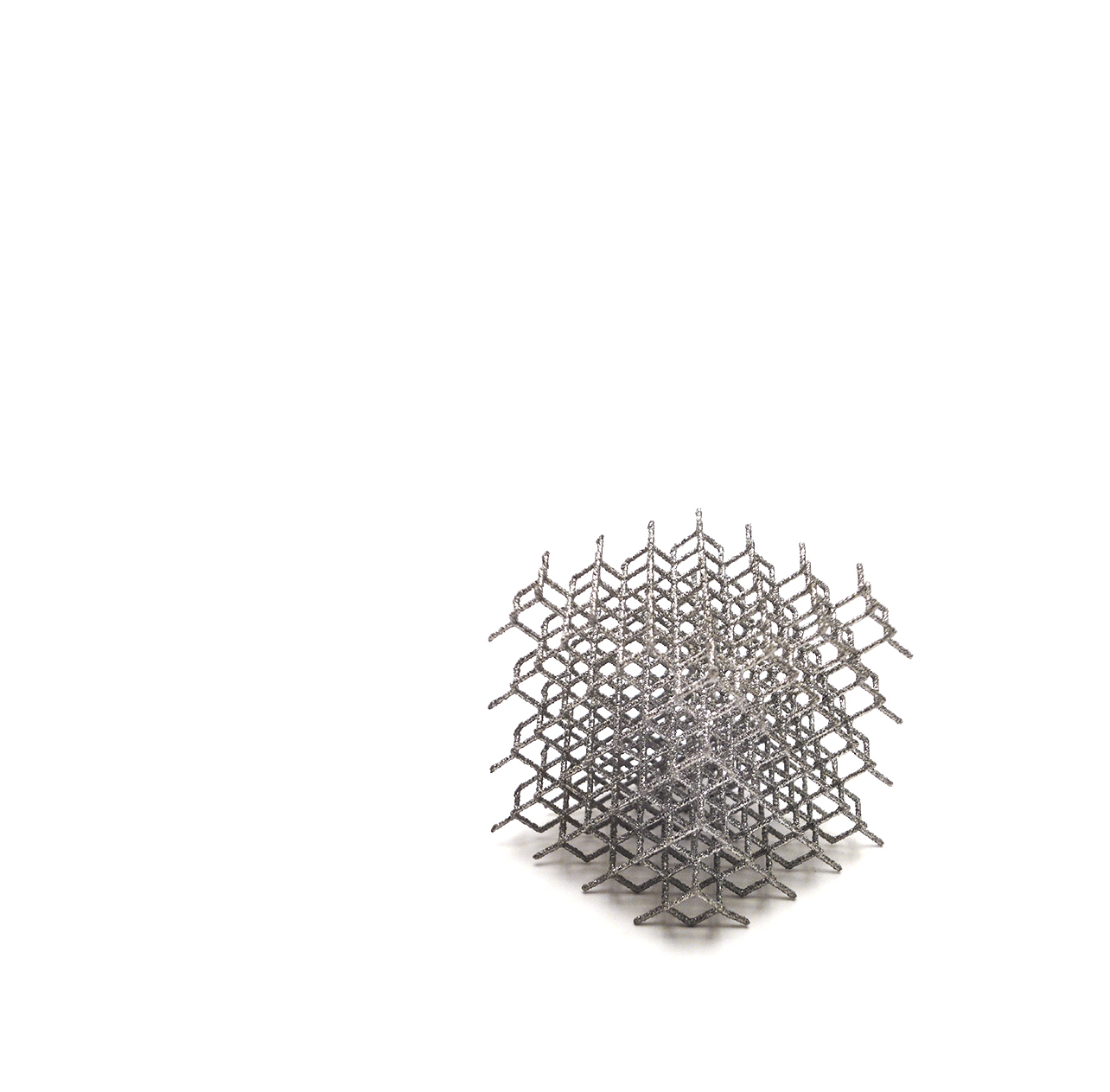
PRIMA TT Glenoid receives CE Mark approval under new European MDR regulation!

We are pleased to announce that the newly developed PRIMA TT Glenoid implant has officially received CE Mark approval under the new European MDR regulation (EU 2017/745). This significant milestone marks a major achievement for us: as a Class III medical device not previously certified under the Medical Device Directive (MDD 93/42/EEC), the PRIMA TT Glenoid's MDR registration signifies our unwavering commitment to the highest standards of safety and quality for patients.
We extend our gratitude to all the teams involved in this successful journey. The company recognizes the dedication and hard work of its employees throughout the rigorous approval process, which played a critical role in bringing this innovative technology to market and ultimately improving patient care in shoulder arthroplasty.