Corporate Governance
We believe in conducting our business in a fair and transparent manner in compliance with the law and the highest ethical standards.

We are a member of Confindustria Dispositivi Medici and we follow its Compliance principles, applying them throughout the entire organization. If Confindustria Dispositivi Medici Compliance Principles are not applicable in some countries, we apply local standards according to the local professional medical association guidelines.

Based on the provision of such documents, we implemented a set of compliance policies and procedures to ensure the lawfulness, correctness, traceability, and transparency of our activities. We appointed a Supervisory Board with the tasks of constant monitoring the adoption and implementation of the compliance procedures, check the company’s activity and notify the Board of Directors of any violation of compliance rules.

In 2018, we established a whistle-blowing mechanism, through our website, which allows any employee to report directly to our Supervisory Body any violation of our compliance policies and procedures to promptly provide an investigation of the situation.
Employees at all levels are regularly and consistently trained on compliance matters both with live courses and by means of a dedicated digital training platform.
Rodrigo Bianchi is a member of the Advisory Board managing and measuring LimaCorporate’s sustainability journey.
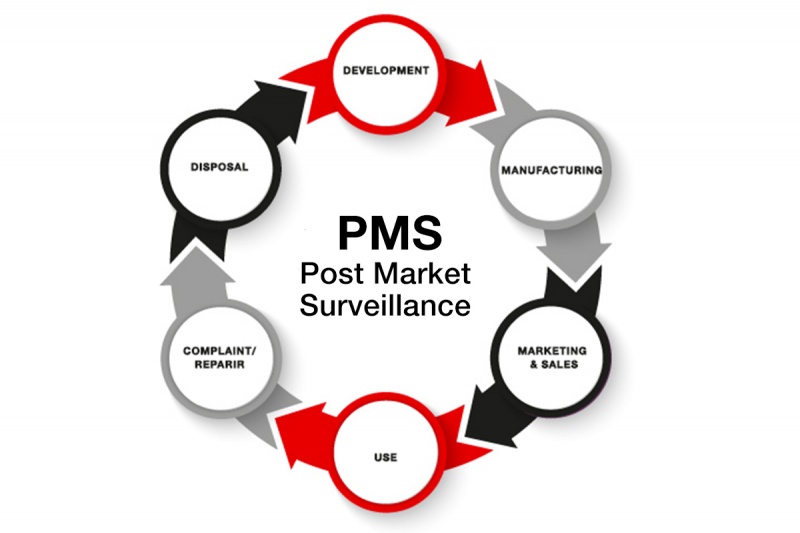
As a medical devices company, we're committed to improving public health ensuring product safety in compliance with industry regulations. We're focused on maintaining a high-quality standard of our implants, and we rely on the post-market surveillance system in place for reactive data gathering and ensuring a proactive evaluation of the information coming from the market.
Post-market surveillance activities monitor the safety and the effectiveness of medical devices after being approved for sale and seeing in-market use.
Among the quality KPIs approved and revised year by year by the Management Team, the ratio between the number of medical complaints received on implantable devices and the number of sold implants (12 months) is constantly under the magnifying glass. Our complaint incidence threshold (internally defined based on the company's years of experience in orthopedics) of 0,06% is not exceeded year by year, quarter by quarter.

To achieve our goal we must choose the most suitable Suppliers in terms of quality of processes and expected delivery deadlines.
Suppliers providing the Company with critical products and services are considered Critical Suppliers and are evaluated on the base of the impact of their supply and their performances on the final product, proportionate to the risk associated with it.
The supplier selection and qualification flow is carried out following a well-structured process according to the high level standards requested by the internal Quality Management System and the main applicable international standards and regulations. Specific practices for monitoring and re-evaluation of the Suppliers are also defined and they include on-site visits and inspections.