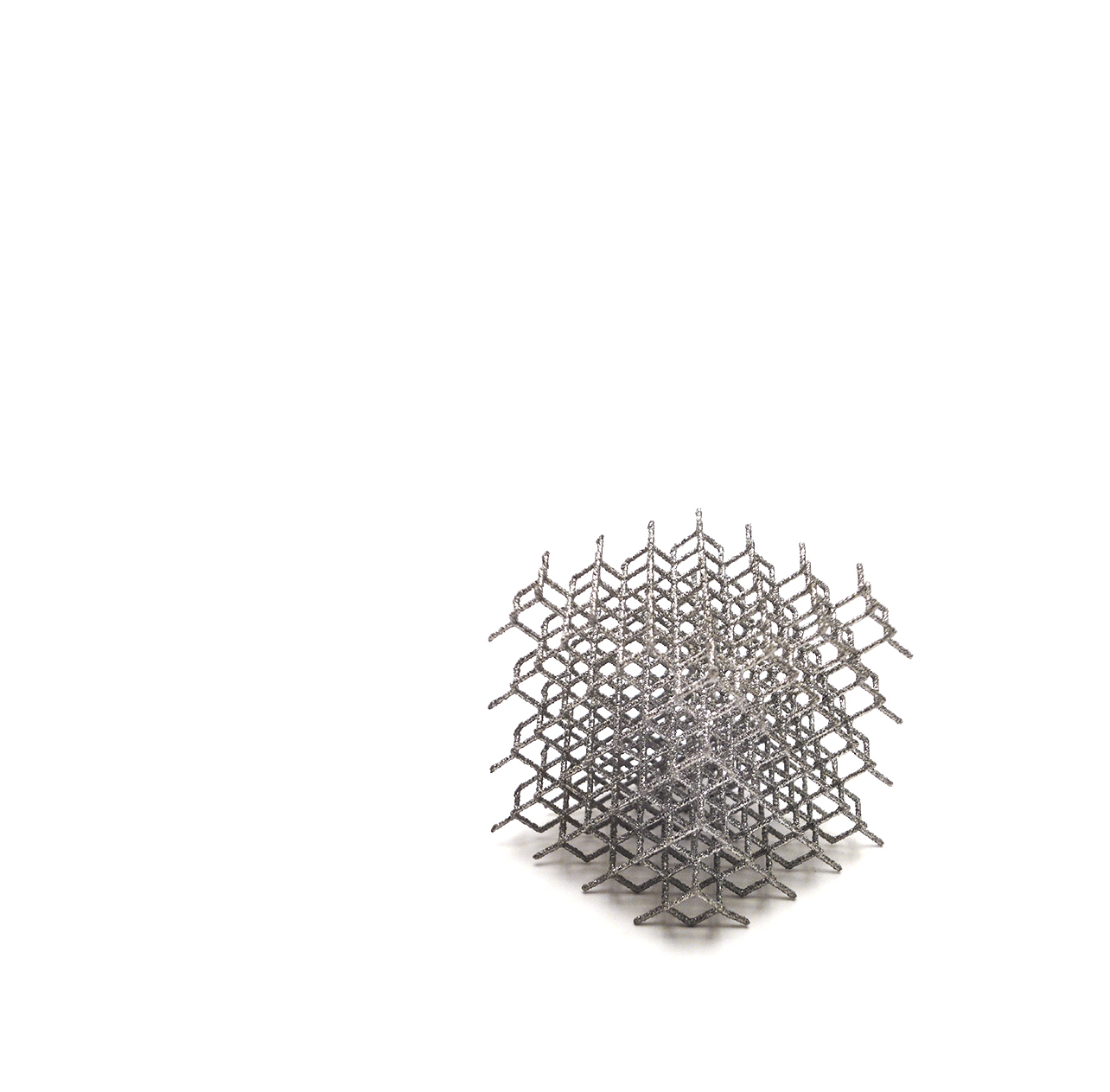
SMR TT Hybrid Glenoid
LimaCorporate’s SMR TT Hybrid Glenoid is the first convertible 3D Printed* Hybrid Glenoid. The central peg is made in LimaCorporate’s patent technology *Trabecular Titanium.
Please note that not all products are available and registered in every market: contact your LimaCorporate Sales Representative for any further information.
Benefits
01
Soft Tissue Management
The wide range of sizes, mismatch options and thicknesses make SMR TT Hybrid Glenoid appropriate for soft tissue balancing.
02
Convertible Glenoid
SMR TT Hybrid Glenoid is invertible to reverse without the central peg removal.
03
Reliable Fixation
The 3D printed Trabecular Titanium central peg enhances primary and biological fixation. Poly peripheral pegs are also cemented.
Images
About
LimaCorporate’s SMR TT Hybrid Glenoid is the first glenoid component with hybrid fixation convertible from anatomic to reverse.
Being part of the SMR System, SMR TT Hybrid Glenoid has been designed to provide solutions for glenoid replacement. It allows soft tissue management, thanks to its wide range of sizes.
The SMR TT Hybrid Glenoid features a polyethylene baseplate connected to a central Trabecular Titanium peg. Using dedicated instruments, the pre-assembled polyethylene baseplate can be disconnected leaving the metal peg in place. A dedicated reverse baseplate can be connected to the central peg.
The use of Trabecular Titanium for the central peg improves stability and tissue ingrowth. The material, the structure, the mechanical properties and the enhanced initial fixation are the premises for an initial primary fixation followed by the biologic integration of the implants [1-5].
See bibliography
Bibliography
[1] A. M. Malhas, J. Granville-Chapman, P. M. Robinson, S. Brookes-Fazakerley, M. Walton, P. Monga, S. Bale, I. Trail Reconstruction of the glenoid using autologous bone-graft and the SMR Axioma TT metal-backed prosthesis. Bone Joint J 2018;100-B:1609–17.
[2] * E. Marin, L. Fedrizzi, M. Regis, M. Pressacco, L. Zagra, S. Fusi. Stability enhancement of prosthetic implants: friction analysis of Trabecular
TitaniumTM. Hip International, 22(4): 427-428, 2012.
[3] * V. Sollazzo, A. Palmieri, L. Massari, F. Carinci. Genetic effects of Trabecular TitaniumTM on cells MG-63 cell line: an in vitro study. J Orthpaed
Traumatol., 13(1): 107, 2012.
[4] * Benazzo F, Botta L, Scaffino MF, Caliogna L, Marullo M, Fusi S, Gastaldi G. Trabecular Titanium can induce in vitro osteogenic differentiation of
human adipose derived stem cells without osteogenic factors. J Biomed Mater Res Part A. 2014:102A:2061–71.
[5] * Devine D, Arens D, Burelli S, Bloch HR, Boure L. In vivo evaluation of the osteointegration of new highly porous Trabecular Titanium™. J Bone
Joint Surg Br. 2012;94-B(Suppl XXXVII):201.
*Results from clinical studies.
[1] A. M. Malhas, J. Granville-Chapman, P. M. Robinson, S. Brookes-Fazakerley, M. Walton, P. Monga, S. Bale, I. Trail Reconstruction of the glenoid using autologous bone-graft and the SMR Axioma TT metal-backed prosthesis. Bone Joint J 2018;100-B:1609–17.
[2] * E. Marin, L. Fedrizzi, M. Regis, M. Pressacco, L. Zagra, S. Fusi. Stability enhancement of prosthetic implants: friction analysis of Trabecular
TitaniumTM. Hip International, 22(4): 427-428, 2012.
[3] * V. Sollazzo, A. Palmieri, L. Massari, F. Carinci. Genetic effects of Trabecular TitaniumTM on cells MG-63 cell line: an in vitro study. J Orthpaed
Traumatol., 13(1): 107, 2012.
[4] * Benazzo F, Botta L, Scaffino MF, Caliogna L, Marullo M, Fusi S, Gastaldi G. Trabecular Titanium can induce in vitro osteogenic differentiation of
human adipose derived stem cells without osteogenic factors. J Biomed Mater Res Part A. 2014:102A:2061–71.
[5] * Devine D, Arens D, Burelli S, Bloch HR, Boure L. In vivo evaluation of the osteointegration of new highly porous Trabecular Titanium™. J Bone
Joint Surg Br. 2012;94-B(Suppl XXXVII):201.
*Results from clinical studies.
Sizing
& Options
Papers
Resources
Disclaimer
Please note that not all products are available and registered in every market. Please contact your LimaCorporate Sales Representative for any further information.
LPSI, SpaceFlex e SYMBOL® are distributed by Limacorporate S.p.A.
BIOLOX® / BIOLOX®delta / BIOLOX OPTION® is a registered trademark of a company of the CeramTec Group, Germany.
LPSI, SpaceFlex e SYMBOL® are distributed by Limacorporate S.p.A.
BIOLOX® / BIOLOX®delta / BIOLOX OPTION® is a registered trademark of a company of the CeramTec Group, Germany.