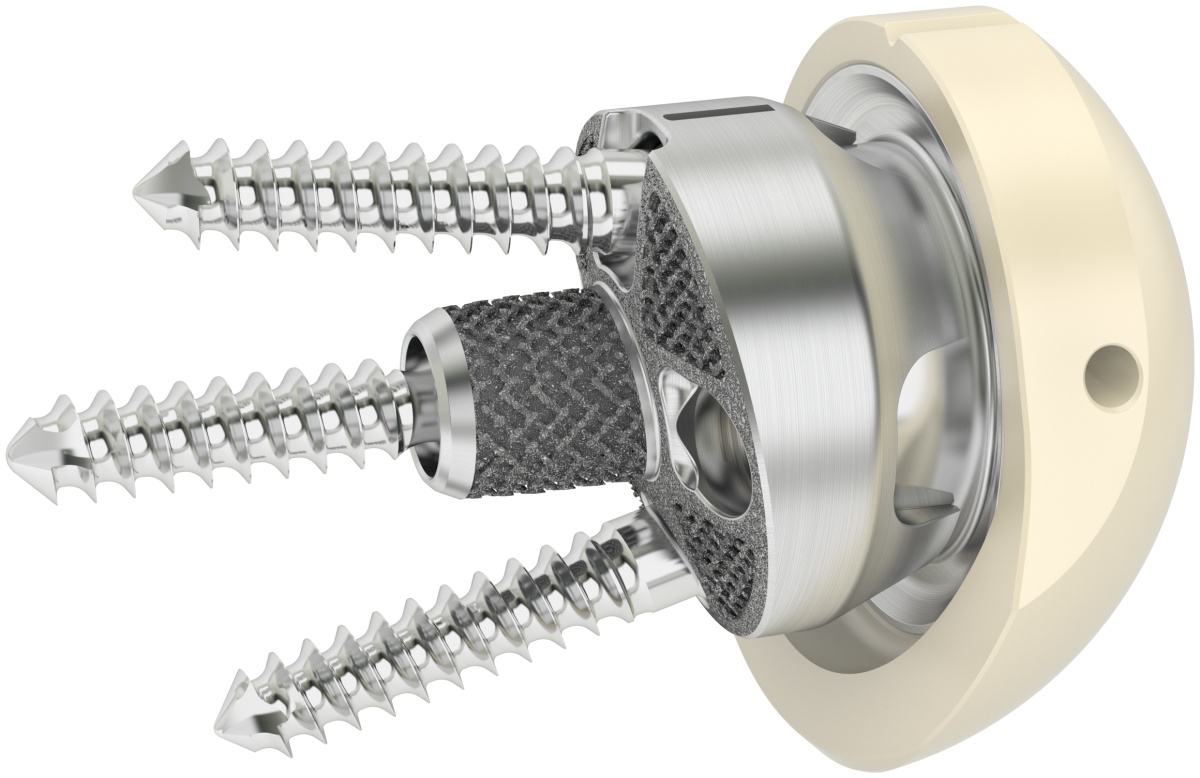
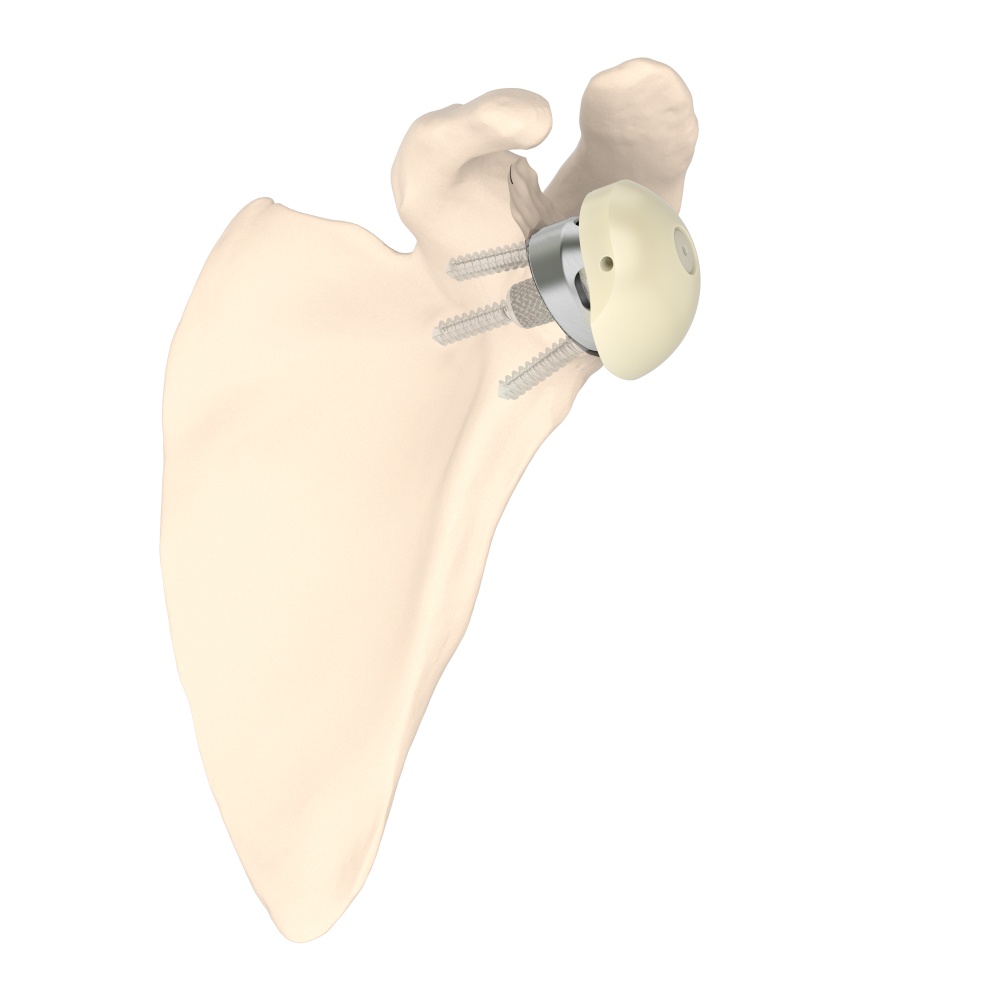
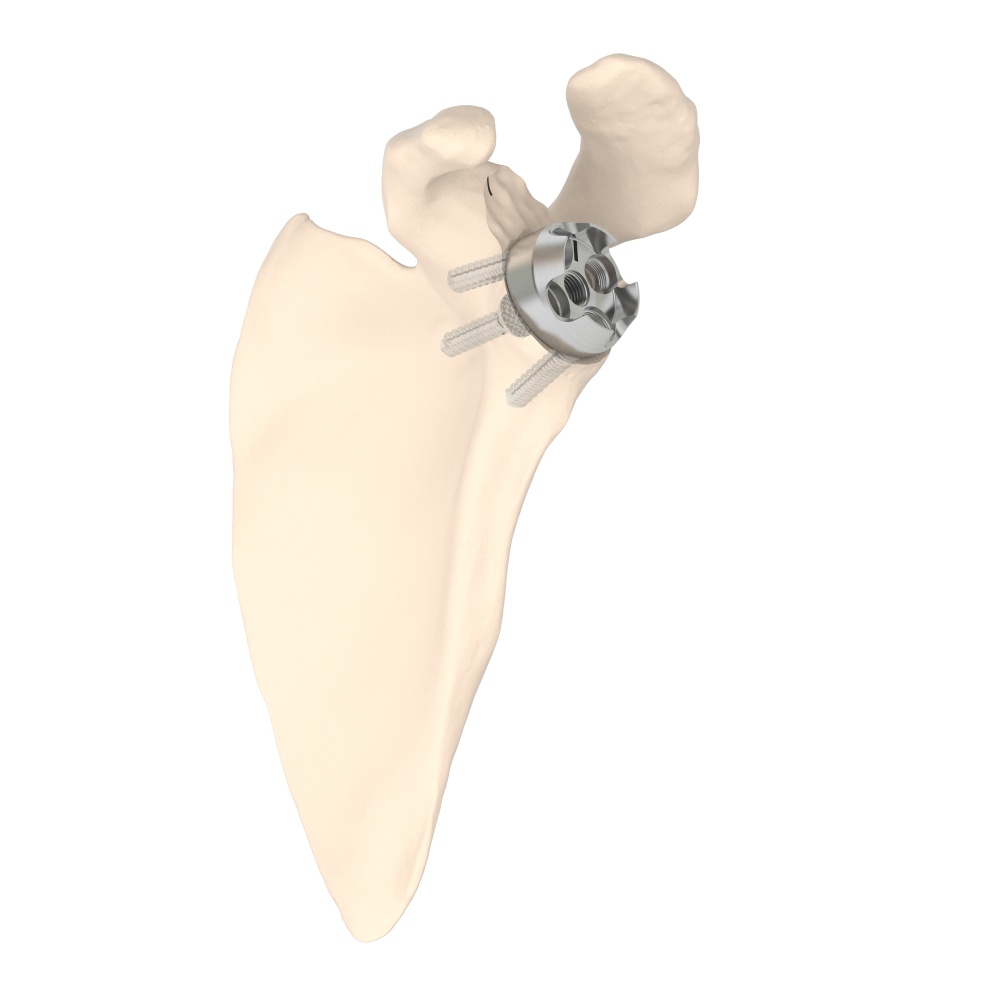
PRIMA TT Glenoid
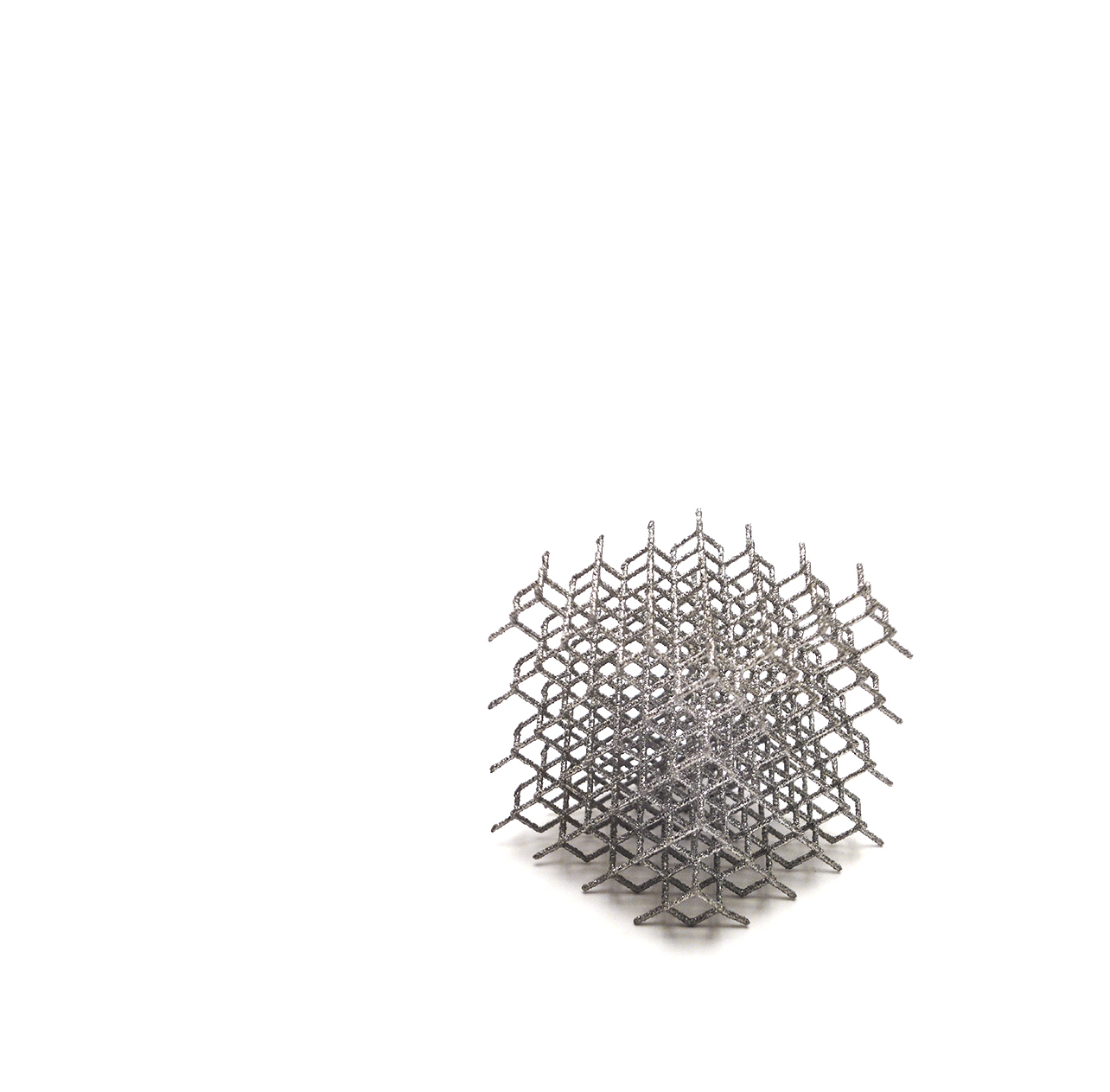
Efficient by DesignPRIMA TT Glenoid consists of a new 3D-printed baseplate designed to match different glenoid morphologies, including several bone loss cases. This implant combines the reliable fixation of TT , a streamlined surgical technique, and the benefits of an advanced planning software.
Benefits
Efficiency
PRIMA is designed to address the need for efficiency of surgeons and OR staff. The optimized ASAP Reamer allows an on-axis streamlined glenoid preparation, enabling surgeons to effectively address the majority of cases with a reduced amount of material.
Technology
It is conceived within the latest technologies. Being a fully 3D-printed off-the-shelf glenoid baseplate, it is designed to be used with advanced planning software to ensure the best match with the glenoid morphology.
Performance
PRIMA TT Glenoid builds on the heritage of TT [1-4] and follows the clinical success of >15.000 SMR TT glenoids implanted worldwide [5-6] . Aware that intraoperative experience is one of the major factors influencing surgeons’ practice, performances have been maximised with a streamlined surgical technique.
About
PRIMA TT Glenoid consists of a new 3D-printed baseplate designed to match different glenoid morphologies, including several bone loss cases, optimizing the surgeon experience.
The user can leverage on the ASAP Reamer & Driller System for a streamlined on-axis glenoid preparation, the support of an advanced planning software, and top-notch materials such as Trabecular Titanium to grant a long-term fixation, proven by its clinical heritage [1-4].
[2]* Sollazzo V, Massari L, Pezzetti F, Girardi A, Farinella F, Lorusso V, Burelli S, Bloch HR, Carinci F. Genetic effects of Trabecular TitaniumTM on MG-63 cell line: a genetic profiling evaluation. ISRN Mater Sci.2011:392763.
[3]* Benazzo F, Botta L, Scaffino MF, Caliogna L, Marullo M, Fusi S, Gastaldi G. Trabecular Titanium can induce in vitro osteogenic differentiation of human adipose derived stem cells without osteogenic factors. J Biomed Mater Res Part A. 2014:102A:2061-71.
[4]* Devine D, Arens D, Burelli S, Bloch HR, Boure L. In vivo evaluation of the osteointegtration of new highly porous Trabecular TitaniumTM. J Bone Joint Surg Br. 2012;94-B(Suppl XXXVII):201.
[5] Malhas AM, Granville-Chapman J, Robinson PM, Brookes-Fazakerly S, Walton M, Monga P, Bale S, Trail I. Reconstruction of the glenoid using autologous bone-graft and the SMR Axioma TT metal-backed prosthesis. Bone Joint J 2018; 100-B:1609-1617.
[6] Cunningham L J, Walton M, Bale S, Trail I A. A prospective radiostereometric analysis of the stability of a metal-backed glenoid component/autograft composite in reverse shoulder arthroplasty. The Bone & Joint Journal 2023; 105-B:912-919.
* Results from pre-clinical study.
LPSI, SpaceFlex e SYMBOL® are distributed by Limacorporate S.p.A.
BIOLOX® / BIOLOX®delta / BIOLOX OPTION® is a registered trademark of a company of the CeramTec Group, Germany.