VRP plate
Anatomical design and optimal biomechanical features to ensure the success of the surgeryVolar Radius PEEK plate
Please note that not all products are available and registered in every market: contact your LimaCorporate Sales Representative for any further information.
Benefits
01
Facilitated rehabilitation
The modulus of elasticity of CFR PEEK close to the cortical bone allows an easier and faster rehabilitation compared to conventional osteosynthesis materials.
02
Optimal revision of the healing process
The radiolucency allows intraoperative control of the reduction of the fracture and better visualization in the post-operative period, avoiding the interference of artefacts in the MRI images.
03
Easy removal
The absence of galvanic corrosion between the plate and screws, together with no excess of the callus on the plate itself allows for easier removal.
Video
Images
About
Anatomical design and optimal biomechanical features to ensure the success of the surgery
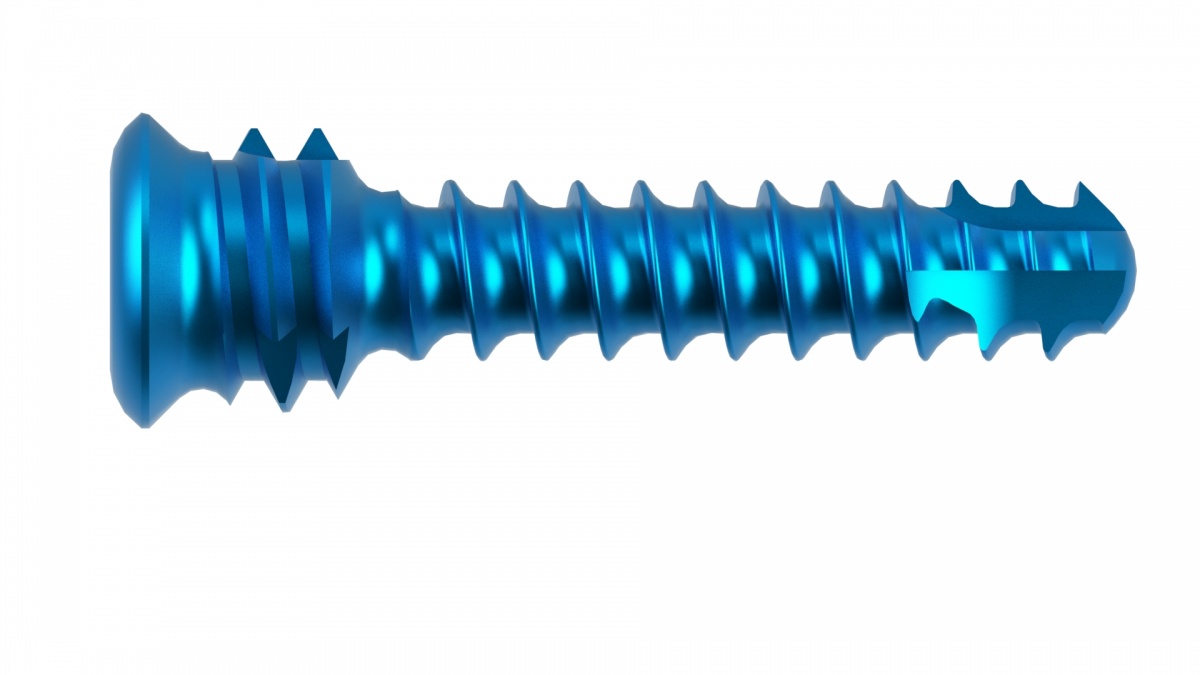
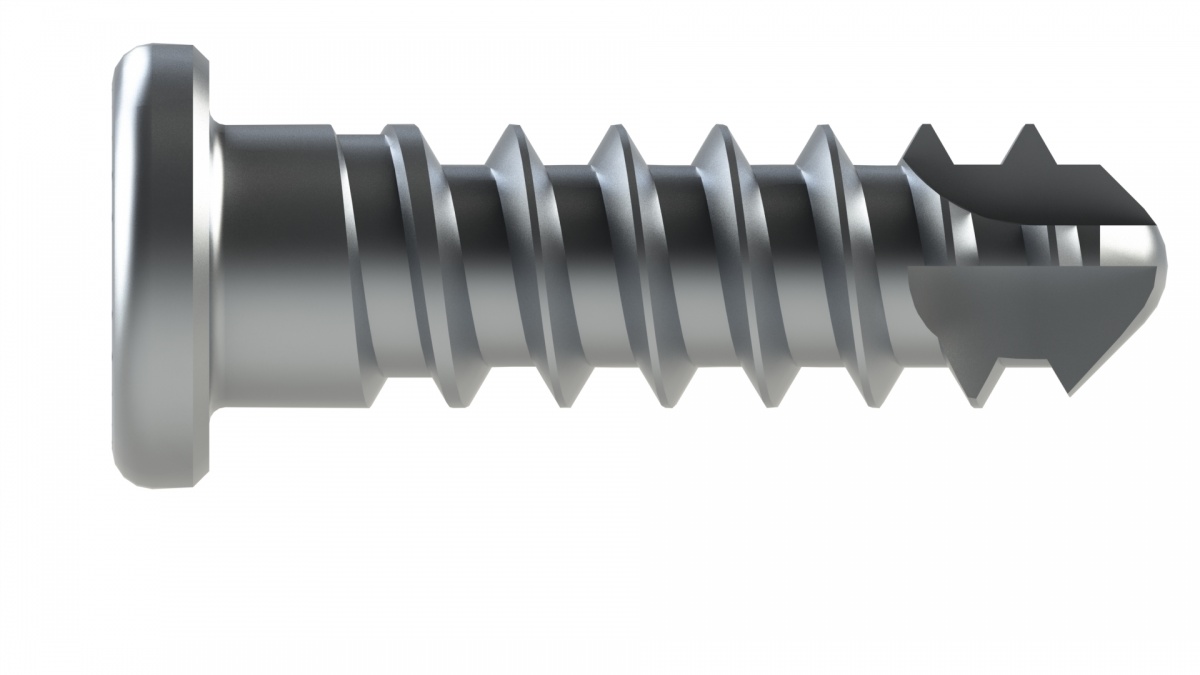
The VRP plate (Volar Radius PEEK plate) is indicated for the treatment of distal radius fractures through a volar approach. The innovative 30% CFR PEEK material displays excellent biomechanical characteristics in terms of elasticity and stabilty, ensuring succesful clinical performance.
The guided insertion procedure of the angular stability screws guarantees perfect control of the positioning of the plate, safeguarding the articular surfaces.
See bibliography
[1] Tarallo at al. Volar PEEK plate for distal radius fracture: analysis of adverse events. European Journal of Orthopaedic Surgery & Traumatology (2020) 30: 1293–1298
[2] Tarallo et al. "A new volar plate made of carbon-fiber-reinforced polyetheretherketon for distal radius fracture: analysis of 40 cases." J Orthop Traumatol. 2014 Dec;15(4):277-283.
[3] Kurtz SM, et al. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials (2007) Nov; 28(32): 4845-69
[2] Tarallo et al. "A new volar plate made of carbon-fiber-reinforced polyetheretherketon for distal radius fracture: analysis of 40 cases." J Orthop Traumatol. 2014 Dec;15(4):277-283.
[3] Kurtz SM, et al. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials (2007) Nov; 28(32): 4845-69
Sizing
& Options
Disclaimer
Please note that not all products are available and registered in every market. Please contact your LimaCorporate Sales Representative for any further information.
LPSI, SpaceFlex e SYMBOL® are distributed by Limacorporate S.p.A.
BIOLOX® / BIOLOX®delta / BIOLOX OPTION® is a registered trademark of a company of the CeramTec Group, Germany.
Implants and instruments are manufactured by LSM-MED S.r.l. and distributed and marketed by Limacorporate S.p.A.
LPSI, SpaceFlex e SYMBOL® are distributed by Limacorporate S.p.A.
BIOLOX® / BIOLOX®delta / BIOLOX OPTION® is a registered trademark of a company of the CeramTec Group, Germany.
Implants and instruments are manufactured by LSM-MED S.r.l. and distributed and marketed by Limacorporate S.p.A.