SMR TT Metal Back
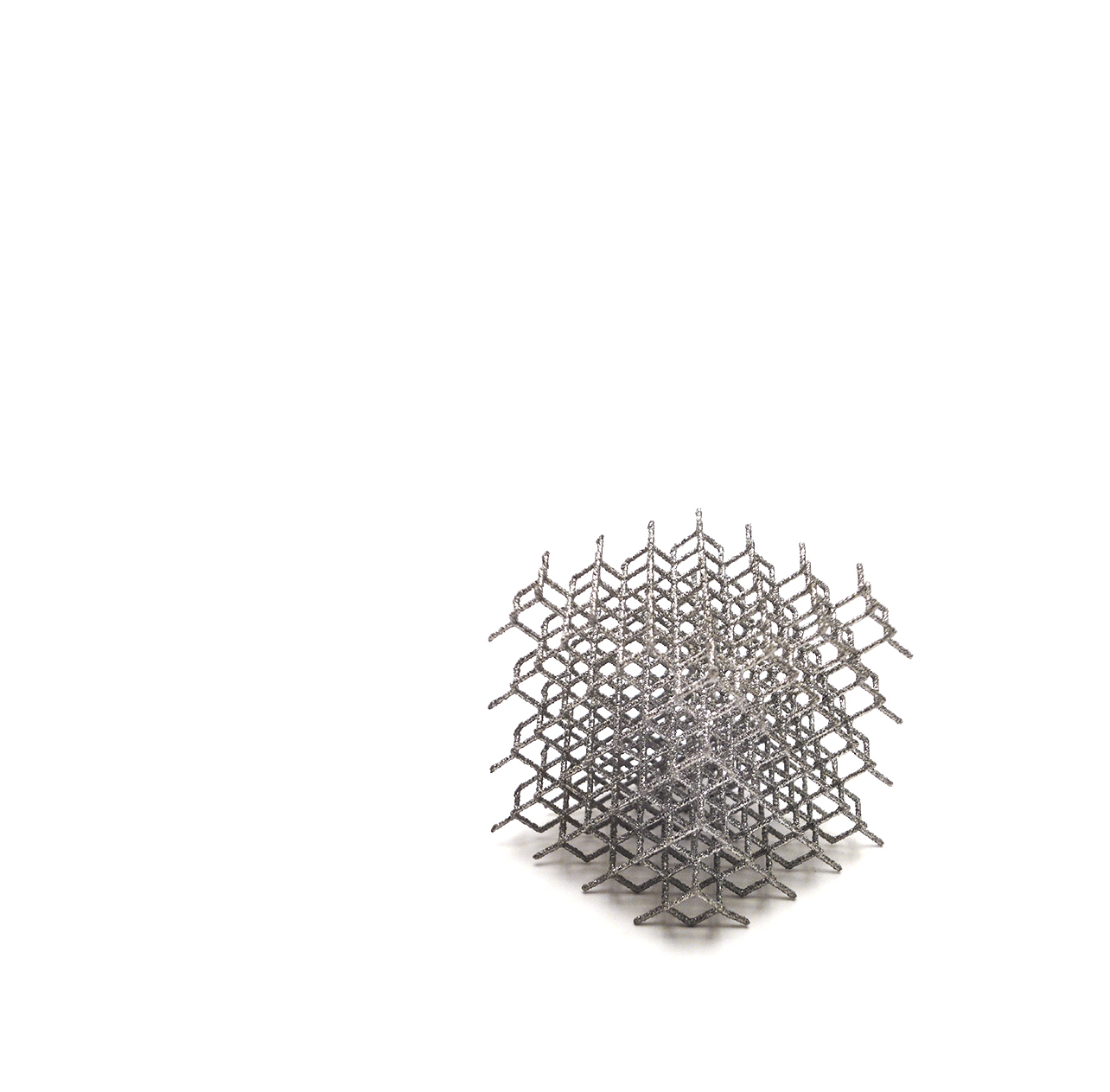
LimaCorporate’s SMR TT Metal Back is a modular glenoid. Its design offers options for deficient glenoids in anatomic and reverse shoulder arthroplasty.
Please note that not all products are available and registered in every market: contact your LimaCorporate Sales Representative for any further information.
Benefits
01
Real Intraoperative Versatility
The SMR TT Metal Back is a universal glenoid allowing orthopedic surgeons to implant a reverse or anatomic prosthesis using reduced instrumentation.
02
Patient Specific Treatment
The extensive modularity of the SMR TT Metal Back offers the possibility to choose the most appropriate solution according to the patient’s unique anatomy.
03
Reliable Fixation
The SMR TT Metal Back implant has been designed to achieve strong primary fixation and osteointegration.
Images
About
Based on clinical heritage of the SMR System [1-7], SMR TT Metal Back [8-9] breaks new ground in glenoid replacement, combining unique implant design with the advanced Trabecular Titanium structure.
The material, the structure, the mechanical properties and the enhanced initial fixation offer greater primary fixation, which favors an improved biologic integration of the implants. An additional instrument set has been developed to allow bone graft preparation. The aim is to restore the joint line by filling in glenoid defects.
Glenoid Bone Graft Instrumentation
The step-by-step guided procedure allows orthopedic surgeons to produce different bone graft sizes adapting to each clinical case.
LimaCorporate’s SMR TT Metal Back offers multiple options of bone graft: straight graft (5, 10 or 15mm) or sloped graft (15° or 20°). The Instrumentation is user-friendly and favors the orthopedic surgeons in a reproducible procedure.
See bibliography
BIBLIOGRAPHY
[1] A. Castagna, M. Randelli, R. Garofalo, L. Maradei, A. Giardella, M. Borroni. Mid-Term results of a metalbacked glenoid component intotal shoulder replacement. J Bone Joint Surg [Br], 92(10): 1410-1415, 2010.
[2] A.Castagna, M. Delcogliano, F. de Caro, G. Ziveri, M. Borroni, S. Gumina, F. Postacchini, C.F. De Biase. Conversion of shoulder arthroplasty to reverse implants: clinical and radiological results using a modular system. Int Orthop. May 2013.
[3] Bloch HR, Budassi P, Bischof A, Agneskirchner J, Domenghini C, Frattini M, Borroni M, Zoni S, Castagna A. Influence of glenosphere design and material on clinical outcomes of reverse total shoulder arthroplasty. Shoulder & Elbow 2014;6:156-64.
[4] S.W. Young, N.M. Everts, C.M. Ball, T.M. Astley, P.C. Poon. The SMR reverse shoulder prosthesis in the treatment of cuffdeficient shoulder conditions. J Shoulder Elbow Surg, 18(4): 622-626, 2009.
[5] A.A. Martinez, A. Calvo, C. Bejarano, I. Carbonel, A. Herrera. The use of the Lima reverse shoulder arthroplasty for the treatment of fracture sequelae of the proximal humerus. J Orthop Sci, 17(2):141-7, 2012.
[6] K. Mohammed, A. Slaven. Reliable osteointegration of a metal back glenoid in conventional total shoulder arthroplasty at minimum 3 years follow up. J Bone Joint Surg Br. 2012; 94-B (Supp XXI): 57-57.
[7] R. Postacchini, A. Castagna, M. Borroni, G. Cinotti, F. Postacchini, S. Gumina. Total shoulder arthroplasty for the treatment of failed hemiarthroplasty in patients with fracture of the proximal humerus. J Shoulder Elbow Surg. 2012 Mar 3.
[8] A. M. Malhas, J. Granville-Chapman, P. M. Robinson, S. Brookes-Fazakerley, M. Walton, P. Monga, S. Bale, I. Trail Reconstruction of the glenoid using autologous bone-graft and the SMR Axioma TT metal-backed prosthesis. Bone Joint J 2018;100-B:1609–17.
[9] Lopiz Y, García-Fernández C, Arriaza A, Rizo B, Marcelo H, Marco F. Midterm outcomes of bone grafting in glenoid defects treated with reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017 Sep;26(9):1581-1588.
[10]* E. Marin, L. Fedrizzi, M. Regis, M. Pressacco, L. Zagra, S. Fusi. Stability enhancement of prosthetic implants: friction analysis of Trabecular TitaniumTM. Hip International, 22(4): 427-428, 2012.
[11]* V. Sollazzo, A. Palmieri, L. Massari, F. Carinci. Genetic effects of Trabecular TitaniumTM on cells MG-63 cell line: an in vitro study. J Orthpaed Traumatol., 13(1): 107, 2012.
[12]* Benazzo F, Botta L, Scaffino MF, Caliogna L, Marullo M, Fusi S, Gastaldi G. Trabecular Titanium can induce in vitro osteogenic differentiation of human adipose derived stem cells without osteogenic factors. J Biomed Mater Res Part A. 2014:102A:2061–71.
[13]* Devine D, Arens D, Burelli S, Bloch HR, Boure L. In vivo evaluation of the osteointegration of new highly porous Trabecular Titanium™. J Bone Joint Surg Br. 2012;94-B(Suppl XXXVII):201.
*Results from clinical studies.
[1] A. Castagna, M. Randelli, R. Garofalo, L. Maradei, A. Giardella, M. Borroni. Mid-Term results of a metalbacked glenoid component intotal shoulder replacement. J Bone Joint Surg [Br], 92(10): 1410-1415, 2010.
[2] A.Castagna, M. Delcogliano, F. de Caro, G. Ziveri, M. Borroni, S. Gumina, F. Postacchini, C.F. De Biase. Conversion of shoulder arthroplasty to reverse implants: clinical and radiological results using a modular system. Int Orthop. May 2013.
[3] Bloch HR, Budassi P, Bischof A, Agneskirchner J, Domenghini C, Frattini M, Borroni M, Zoni S, Castagna A. Influence of glenosphere design and material on clinical outcomes of reverse total shoulder arthroplasty. Shoulder & Elbow 2014;6:156-64.
[4] S.W. Young, N.M. Everts, C.M. Ball, T.M. Astley, P.C. Poon. The SMR reverse shoulder prosthesis in the treatment of cuffdeficient shoulder conditions. J Shoulder Elbow Surg, 18(4): 622-626, 2009.
[5] A.A. Martinez, A. Calvo, C. Bejarano, I. Carbonel, A. Herrera. The use of the Lima reverse shoulder arthroplasty for the treatment of fracture sequelae of the proximal humerus. J Orthop Sci, 17(2):141-7, 2012.
[6] K. Mohammed, A. Slaven. Reliable osteointegration of a metal back glenoid in conventional total shoulder arthroplasty at minimum 3 years follow up. J Bone Joint Surg Br. 2012; 94-B (Supp XXI): 57-57.
[7] R. Postacchini, A. Castagna, M. Borroni, G. Cinotti, F. Postacchini, S. Gumina. Total shoulder arthroplasty for the treatment of failed hemiarthroplasty in patients with fracture of the proximal humerus. J Shoulder Elbow Surg. 2012 Mar 3.
[8] A. M. Malhas, J. Granville-Chapman, P. M. Robinson, S. Brookes-Fazakerley, M. Walton, P. Monga, S. Bale, I. Trail Reconstruction of the glenoid using autologous bone-graft and the SMR Axioma TT metal-backed prosthesis. Bone Joint J 2018;100-B:1609–17.
[9] Lopiz Y, García-Fernández C, Arriaza A, Rizo B, Marcelo H, Marco F. Midterm outcomes of bone grafting in glenoid defects treated with reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017 Sep;26(9):1581-1588.
[10]* E. Marin, L. Fedrizzi, M. Regis, M. Pressacco, L. Zagra, S. Fusi. Stability enhancement of prosthetic implants: friction analysis of Trabecular TitaniumTM. Hip International, 22(4): 427-428, 2012.
[11]* V. Sollazzo, A. Palmieri, L. Massari, F. Carinci. Genetic effects of Trabecular TitaniumTM on cells MG-63 cell line: an in vitro study. J Orthpaed Traumatol., 13(1): 107, 2012.
[12]* Benazzo F, Botta L, Scaffino MF, Caliogna L, Marullo M, Fusi S, Gastaldi G. Trabecular Titanium can induce in vitro osteogenic differentiation of human adipose derived stem cells without osteogenic factors. J Biomed Mater Res Part A. 2014:102A:2061–71.
[13]* Devine D, Arens D, Burelli S, Bloch HR, Boure L. In vivo evaluation of the osteointegration of new highly porous Trabecular Titanium™. J Bone Joint Surg Br. 2012;94-B(Suppl XXXVII):201.
*Results from clinical studies.
Papers
Resources
Disclaimer
Please note that not all products are available and registered in every market. Please contact your LimaCorporate Sales Representative for any further information.
LPSI, SpaceFlex e SYMBOL® are distributed by Limacorporate S.p.A.
BIOLOX® / BIOLOX®delta / BIOLOX OPTION® is a registered trademark of a company of the CeramTec Group, Germany.
LPSI, SpaceFlex e SYMBOL® are distributed by Limacorporate S.p.A.
BIOLOX® / BIOLOX®delta / BIOLOX OPTION® is a registered trademark of a company of the CeramTec Group, Germany.