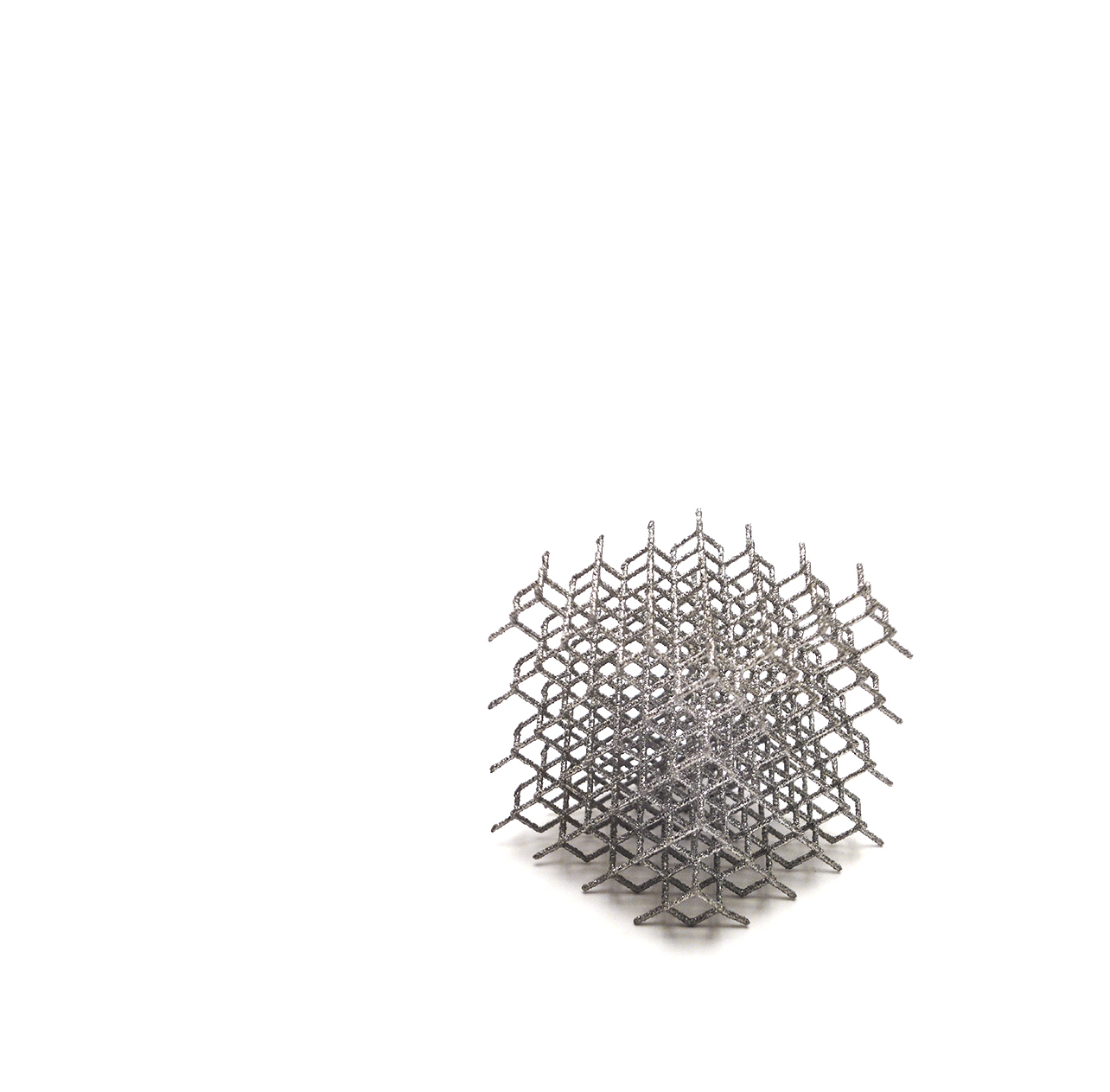
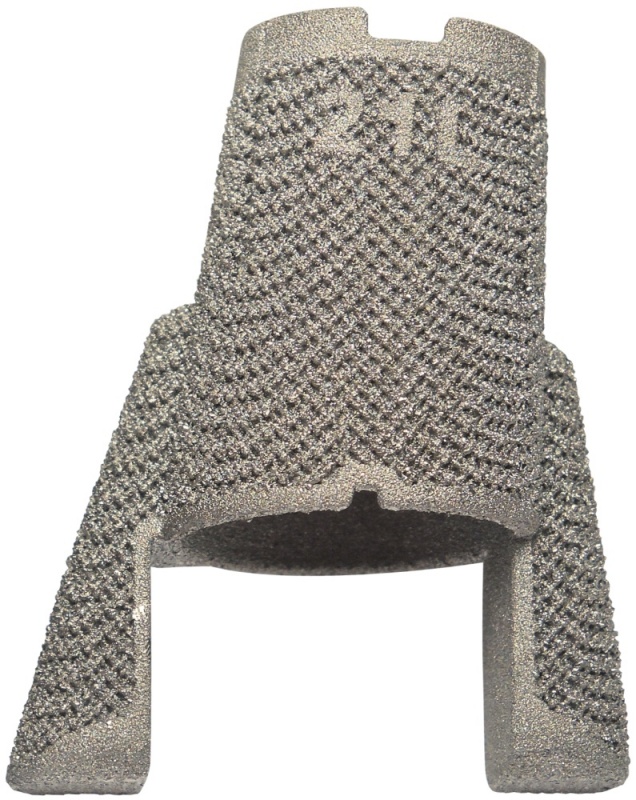
AMF TT Cones
AMF TT Cones are intended for use in skeletally mature patients with bone defects or poor bone quality (osteoporotic bone) or in case of sclerotic bone that requires supplemental metaphyseal fixation in the clinical judgment of the surgeon. The AMF TT Cones are available in four versions: + Central Femur / + Bicondylar Femur / + Central Tibia / + Peripheral Tibia
Benefits
AMF means Anatomic Metaphyseal Fixation
+ Anatomical design to fit the femoral and tibial anatomy + Data from anatomical studies in cooperation with TechMah
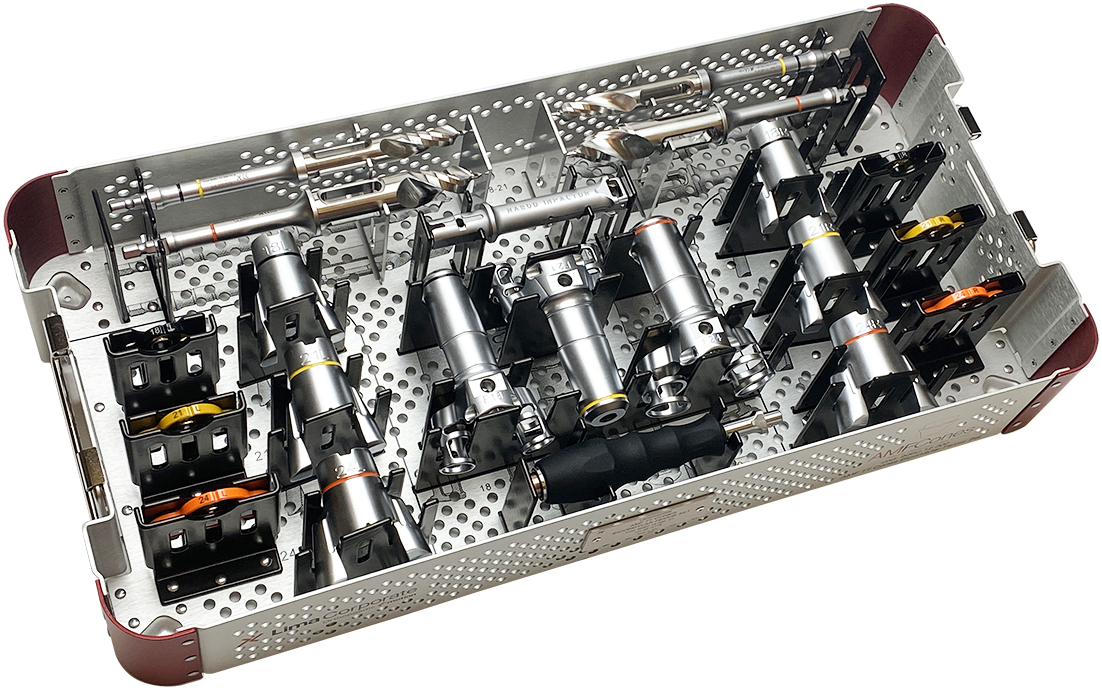
CONTROL with ream only preparation
+ State of the Art Instruments + Completely guided ream only preparation + Streamlined trays for simple workflow
CONFIDENCE from over a decade of clinically proven innovation in 3D printing
+ Reliable FIXATION [2,3,6] + Biological FEATURES [1,7,8] + Proven PERFORMANCE [1-15]
About
The AMF TT Cones are offered in 4 constructs: Central Femur, Bicondylar Femur, Central Tibia, and Peripheral Tibia.
The AMF TT Cones are knee prosthesis components intended to be used to:
+ reinforce the medullary cavity of the tibia and femur
+ fill a proximal tibial or distal femoral defect that may result from the removal of a primary knee system
+ provide support to the tibial baseplate or femoral component by means of bone cement
They are used in combination with the Physica cemented tibial plate with the Physica PS femoral component and Multigen Plus CCK and H Systems, which are manufactured by LimaCorporate.
AMF TT Cones are intended for uncemented fixation to the bone and are fixed to the femoral and tibial implants using bone cement.
[2] Yoshimoto K, Nakashima Y, Wakiyama M, Hara D, Nakamura A, Iwamoto M. Initial stability of a highly porous titanium cup in an acetabular bone defect model. J Orthop Sci. 2018 Jul;23(4):665-670.
[3] De Martino I, Sculco P, Meyers K, Nocon A, Wright T, Sculco T. Initial Stability in Highly Porous Metal Acetabular Cups: A Biomechanical Study. In: Proceedings of 29th Annual Congress of the International Society for Technology in Arthroplasty (ISTA); 2016 October 5-8; Boston, USA.
[4] Singh J, Odak S, Neelakandan K, Walton MJ, Monga P, Bale S, Trail I. Survivorship of autologous structural bone graft at a minimum of 2 years when used to address significant glenoid bone loss in primary and revision shoulder arthroplasty: a computed tomographic and clinical review. J Shoulder Elbow Surg. 2021 Mar;30(3):668-678.
[5] National Joint Registry for England, Wales and Northern Ireland (NJR). Implant Summary Report on DELTA-TT Cups — Hip Primary Implants. Hemel Hempstead (UK): NJR Centre. 2021 Feb.Contact for more information: clinical.research@limacorporate.com
[6] Marin E, Fedrizzi L, Regis M, Pressacco M, Zagra L, Fusi S. Stability Enhancement Of Prosthetic Implants: Friction Analysis Of Trabecular Titanium. Hip Int. 2012;22(04):427-428.
[7] Bondarenko S, Dedukh N, Filipenko V, Akonjom M, Badnaoui AA, Schwarzkopf R. Comparative analysis of osseointegration in various types of acetabular implant materials. Hip Int. 2018 Nov;28(6):622-628.
[8] Dall’Ava L, Hothi H, Henckel J, Di Laura A, Tirabosco R, Eskelinen A, Skinner J, Hart A. Osseointegration of retrieved 3D-printed, off-the-shelf acetabular implants. Bone Joint Res. 2021 Jul;10(7):388-400.
[9] Perticarini L, Zanon G, Rossi SM, Benazzo FM. Clinical and radiographic outcomes of a Trabecular Titanium™ acetabular component in hip arthroplasty: results at minimum 5 years follow-up. BMC Musculoskelet Disord. 2015 Dec 3;16:375.
[10] Massari L, Bistolfi A, Grillo PP, Borré A, Gigliofiorito G, Pari C, Francescotto A, Tosco P, Deledda D, Ravera L, Causero A. Periacetabular Bone Densitometry After Total Hip Arthroplasty with Highly Porous Titanium Cups: A Two-Year Follow-Up Prospective Study. Hip Int. 2017;27(6):551-7.
[11] Steno B, Kokavec M, Necas L. Acetabular revision arthroplasty using trabecular titanium implants. Int Orthop. 2015 Mar;39(3):389-95.
[12] De Meo F, Cacciola G, Bellotti V, Bruschetta A, Cavaliere P. Trabecular Titanium acetabular cups in hip revision surgery: mid-term clinical and radiological outcomes. Hip Int. 2018 December;28(S2):61–5.
[13] Munegato D, Bigoni M, Sotiri R, Bruschetta A, Omeljaniuk RJ, Turati M, Rossi A, Zatti G. Clinical and radiological outcomes of acetabular revision with the Delta Revision TT cup. Hip Int. 2018;28(S2):54–60.
[14] Sollazzo V, Massari L, Pezzetti F, Girardi A, Farinella F, Lorusso V, Burelli S, Bloch HR, Carinci F. Genetic effects of Trabecular Titanium™ on MG-63 cell line: a genetic profiling evaluation. ISRN Mater Sci. 2011:392763.
[15] Marin E, Fusi S, Pressacco M, Paussa L, Fedrizzi L. Characterization of cellular solids in Ti6Al4V for orthopaedic implant applications: Trabecular Titanium. J Mech Behav Biomed Mater. 2010 Jul;3(5):373–81.
Sizing
& Options
LPSI, SpaceFlex e SYMBOL® are distributed by Limacorporate S.p.A.
BIOLOX® / BIOLOX®delta / BIOLOX OPTION® is a registered trademark of a company of the CeramTec Group, Germany.